*ESL:Electrostatic Levitation
Thermophysical property measurements of undercooled melts using electrostatic levitation method.
Electrostatic levitation method utilizes the Coulomb force between a charged sample and surrounding electrodes and levitates the sample against gravity. This levitation method needs a high speed feedback control system to stably levitate a sample.
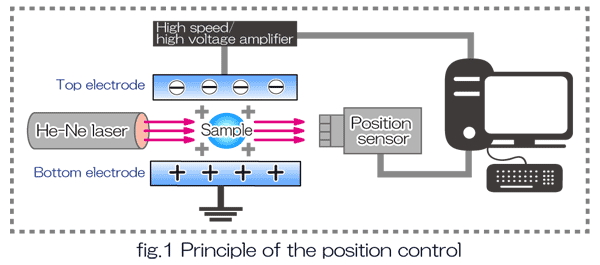
Fig.1 depicts the control system of the ESL. A collimated laser beam projects a sample shadow in the position sensor, and position data (vertical and horizontal position) are fed to a computer where PID (proportional, integral, differential) calculation is executed. By shooting high power laser beams to the stably levitated sample, the sample can be molten without crucibles. This containerless processing technique is very useful to handle liquid metals, which are chemically active in high temperature and easily react with containers.
Moreover, since heterogeneous nucleation is suppressed without crucibles, samples can be easily brought to undercooled temperature region.
Electrostatic levitator in Ishikawa-lab
 fig.2-1
fig.2-1(1) Overview
Chamber size: 400mm in diameter
Vacuum level: 10-5 Pa
Number of optical windows: around 20
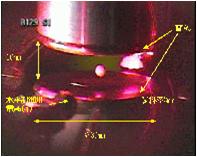 fig.2-2
fig.2-2(2)Electrodes
Top, bottom, and 4 side electrodes
distance of Top and Bottom electrode:10mm
Diameter of bottom electrode :30mm
sample size: around 2mm
 fig.2-3
fig.2-3(3)Magnified sample image (molten tungsten)
tungsten has the highest melting temperature among metal elements. More than 500 W laser power is needed to melt this sample.
Thermophysical property measurement using the Electrostatic levitator.
From electrostatically levitated samples, several thermophysical properties can be obtained.
Density
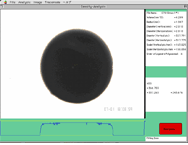 fig.3-1
fig.3-1Since molten samples become spherical, volume of the samples can be measured using computer based image analysis. Still images during sample cooling are obtained from video and analyzed.
Constant pressure heat capacity
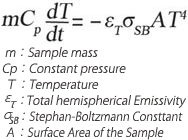 equation(1)
equation(1)In high vacuum condition, the levitated sample is cooled down only by radiation when heating lasers are turned off. Temperature changes during this radiative cooling can be described in equation (1), and temperature data measured by pyrometer is shown in Fig. 3-2.
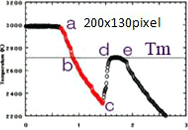 Fig.3-2
Fig.3-2From point "a" to point "c", dT/dt can be calculated and Cp/ετ can be obtained. In order to get Cp, another measurement technique to directly measure ετ is needed.
Surface tension and viscosity
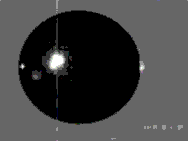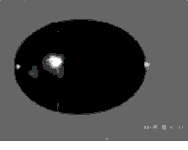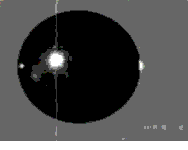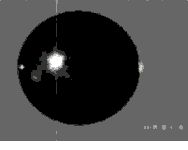 Fig.3-3(a) Mode-2 Oscillation
Fig.3-3(a) Mode-2 Oscillationof a levitated sample
Surface tension and viscosity can be measured simultaneously by oscillation drop technique. In this technique, mode-2 oscillation (shown in the movie) is excited on the levitated sample.
» Mode-2 Oscillation of a levitated sample (MPEG)
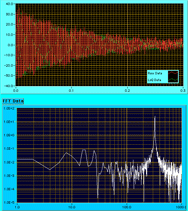 Fig.3-3(b)
Fig.3-3(b) Fig. 3-3(b) shows the fluctuation of vertical diameter of the levitated sample (red) after the drop oscillation, and result of FFT analysis. From the peak frequency ω (determined by the FFT) and decay constant τ (determined from red signal), surface tension γ and viscosity η can be calculated using the following equations.
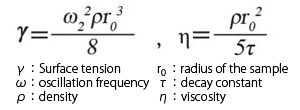
Improvement of viscosity measurement
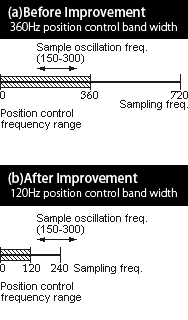 Fig.3-4
Fig.3-4It was found that the sample position control affects the free decay of sample oscillation which results in erroneous viscosity measurements.
When the sample positon control frequency range overlaps with the sample oscillation frequency, the position control excite or suppress sample oscillation.
Viscosity data has been re- measured with the improved measurement procedure in which sample position control frequency was reduced to avoid the above mentioned overlap.
Re-measured sample :Ti,Ni,Zr,Nb,Mo,Ru,Rh,Tb,Hf,Ta,W,Re,Os,Ir, and Pt
※Data can be seen in the Result of thermophysical property measurement of refractory metals.
Electrostatic Levitation Furnace under Pressurized gaseous condition (PELF)
Pressurized version of electrostatic levitator is used mainly for oxide samples, which are too evaporative to keep their stoichiometry under high vacuum. Chamber pressure can be increased up to 4 atm., with dry air or nitrogen.





